《TAIPEI TIMES》 FDA recalls six drugs amid scare
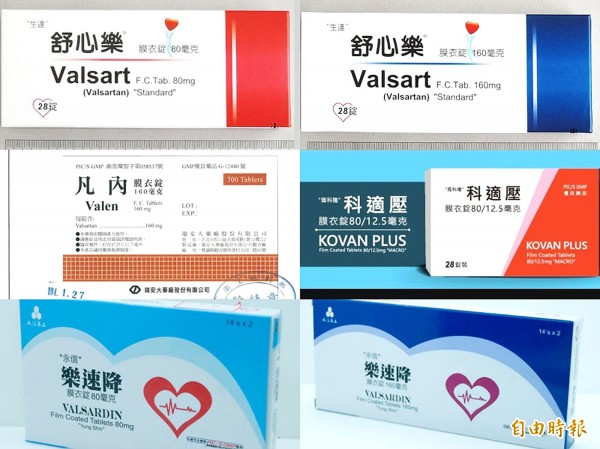
The prescription drugs for treating heart disease and hypertension that are being recalled over concerns that they could contain a probable carcinogen. Photo: CNA
ABOUT-TURN: Despite earlier saying that Taiwan was not affected, the FDA has found six products that used valsartan produced by the Chinese company involved
By Lee I-chia / Staff reporter
Six types of prescription drugs for treating heart disease and hypertension registered under Taiwanese pharmaceutical companies are being recalled as a precaution, as they could contain a probable carcinogen, the Food and Drug Administration (FDA) said yesterday.
The announcement came after governments in Hong Kong, Macau, the UK and several other countries ordered recalls of anti-hypertensive drugs containing valsartan produced by Zhejiang Huahai Pharmaceutical Co Ltd (浙江華海製藥公司) in China.
N-nitrosodimethylamine, a chemical classified as a probable human carcinogen based on laboratory test results, was detected in the valsartan.
The FDA on Saturday said that the recalled products registered under pharmaceutical company Actavis Generics were not imported to Taiwan.
However, after a preliminary examination of drugs containing valsartan that are registered in Taiwan, it discovered that six products contain valsartan from Huahai Pharmaceutical Co.
The products being recalled are: Valsart FC Tablet 160mg and Valsart FC Tablet 80mg registered to Standard Chemical and Pharmaceutical Co Ltd (生達化學製藥); Kovan Plus Coated Tablets 80/12.5mg registered to Macro Co Ltd (瑪科隆); Valsardin Film Coated Tablet 160mg and Valsardin Film Coated Tablet 80mg registered to Yung Shin Pharmaceutical Industrial Co (永信藥品); and Valen FC Tablet 160mg registered to Purzer Pharmaceutical Co Ltd (瑞安大藥廠).
The six products that are being recalled account for about 3 to 4 percent of their category in Taiwan, so the majority of heart disease patients do not have to panic, FDA official Chi Jo-feng (祁若鳳) said.
Healthcare facilities and pharmacies have been told to recall the products within a month, the FDA said, adding that patients taking the recalled drugs should not to stop taking them, but rater see a doctor as soon as possible for an alternative prescription.
新聞來源:TAIPEI TIMES


















